Microneedle Cosmetic Delivery Systems in 2025: Transforming the Future of Skincare Delivery. Explore Breakthrough Technologies, Market Expansion, and What’s Next for This Rapidly Growing Industry.
- Executive Summary: Key Trends and Market Drivers in 2025
- Market Size and Forecast (2025–2030): Growth Projections and CAGR Analysis
- Technological Innovations: Next-Gen Microneedle Designs and Materials
- Competitive Landscape: Leading Companies and Strategic Partnerships
- Regulatory Environment: Global Standards and Compliance Challenges
- Consumer Adoption: Shifting Preferences and Demand Drivers
- Application Areas: Skincare, Anti-Aging, and Beyond
- Supply Chain and Manufacturing Advances
- Sustainability and Biocompatibility in Microneedle Systems
- Future Outlook: Opportunities, Challenges, and Strategic Recommendations
- Sources & References
Executive Summary: Key Trends and Market Drivers in 2025
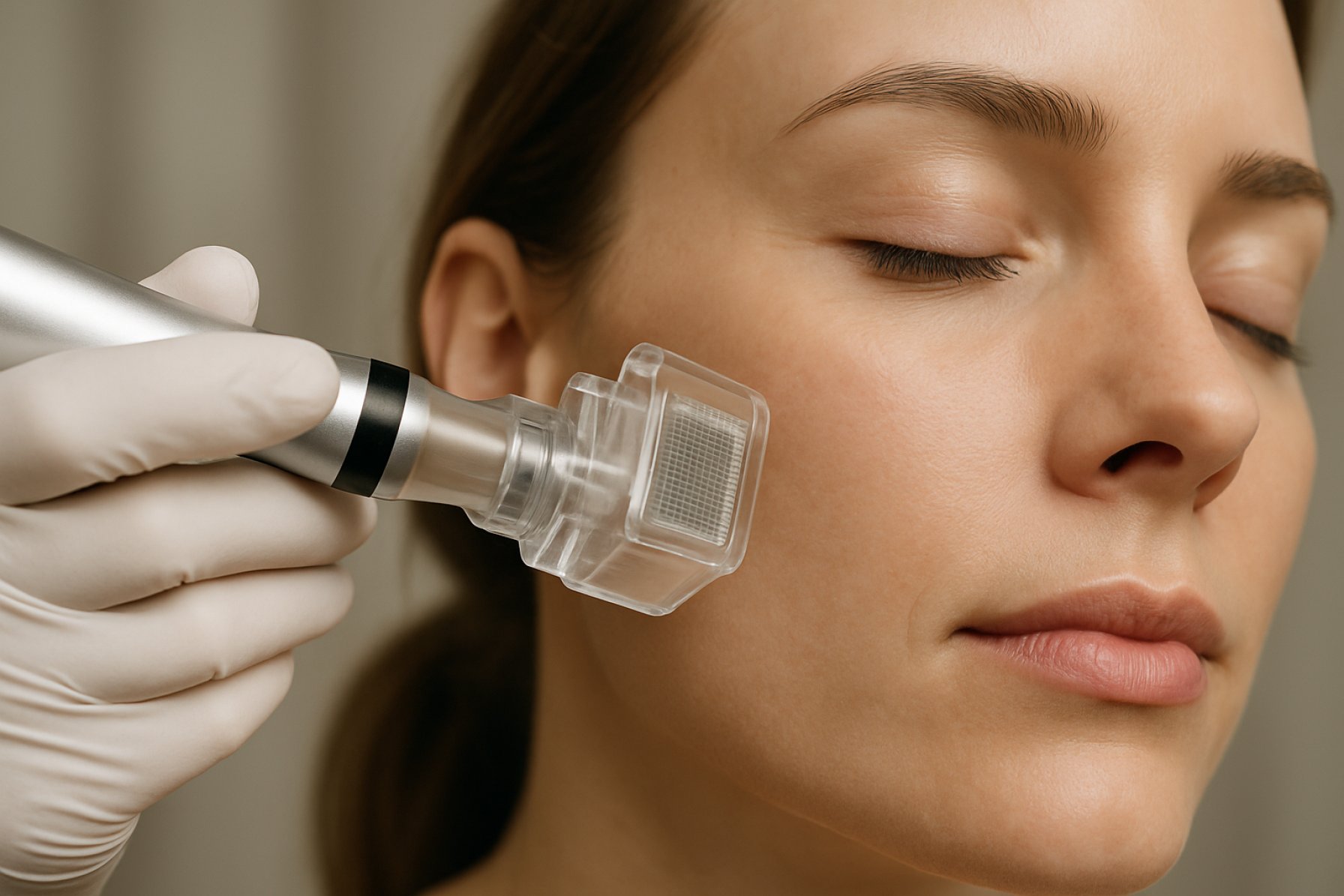
Microneedle cosmetic delivery systems are poised for significant growth and innovation in 2025, driven by consumer demand for minimally invasive, effective, and convenient skincare solutions. These systems, which utilize arrays of microscopic needles to enhance transdermal delivery of active ingredients, are increasingly being adopted in both professional and at-home cosmetic applications. The convergence of advanced materials science, regulatory clarity, and shifting consumer preferences is accelerating the commercialization and acceptance of microneedle-based products.
Key industry players are expanding their portfolios and scaling up production to meet rising demand. 3M, a pioneer in microneedle technology, continues to leverage its expertise in microfabrication and drug delivery to develop new cosmetic applications, including anti-aging and skin-brightening patches. LTS Lohmann Therapie-Systeme AG, known for its transdermal systems, is actively collaborating with cosmetic brands to adapt its microneedle platforms for skincare actives such as hyaluronic acid and peptides. Meanwhile, NanoPass Technologies is expanding its microneedle manufacturing capabilities, targeting both medical and cosmetic markets with its silicon-based microneedle arrays.
The market is also witnessing the entry of major beauty conglomerates. L'Oréal has announced ongoing research and pilot launches of microneedle patches for targeted skincare, leveraging its global R&D infrastructure to accelerate product development. Similarly, Shiseido Company, Limited is investing in microneedle patch technology, focusing on anti-wrinkle and brightening solutions tailored for Asian markets. These initiatives are supported by a growing body of clinical evidence demonstrating the efficacy and safety of microneedle-based delivery for cosmetic actives.
Regulatory agencies in key markets, including the U.S. Food and Drug Administration and the European Medicines Agency, are providing clearer guidance on the classification and approval pathways for microneedle cosmetic products, reducing barriers to market entry and fostering innovation. This regulatory clarity is expected to further boost investment and partnerships between technology developers and established cosmetic brands.
Looking ahead, the outlook for microneedle cosmetic delivery systems in 2025 and beyond is robust. The sector is expected to benefit from continued advancements in biocompatible materials, precision manufacturing, and personalized skincare formulations. As consumer awareness grows and product offerings diversify, microneedle systems are set to become a mainstream modality in the global cosmetic industry, offering new opportunities for brands and improved outcomes for consumers.
Market Size and Forecast (2025–2030): Growth Projections and CAGR Analysis
The global market for microneedle cosmetic delivery systems is poised for robust growth between 2025 and 2030, driven by increasing consumer demand for minimally invasive aesthetic procedures and advancements in transdermal delivery technologies. As of 2025, the market is characterized by a surge in product launches, regulatory approvals, and strategic collaborations among leading device manufacturers and cosmetic brands. The adoption of microneedle patches and rollers for applications such as anti-aging, skin rejuvenation, and targeted delivery of active ingredients is expanding rapidly, particularly in North America, Europe, and Asia-Pacific.
Key industry players such as 3M, a pioneer in microneedle technology, continue to invest in research and development to enhance the efficacy and safety of their cosmetic delivery platforms. 3M’s expertise in microfabrication and polymer science has enabled the commercialization of dissolvable and solid microneedle arrays tailored for cosmetic use. Similarly, LTS Lohmann Therapie-Systeme AG is expanding its portfolio of microneedle patches, leveraging its established transdermal delivery capabilities to address the growing demand for non-invasive cosmetic solutions.
In Asia, companies such as CosMED Pharmaceutical Co., Ltd. and Nissha Co., Ltd. are at the forefront of innovation, introducing microneedle products for home-use and professional skincare markets. These firms are capitalizing on the region’s strong beauty and personal care sector, with Japan and South Korea emerging as key hubs for product development and commercialization.
Market growth is further supported by the increasing availability of over-the-counter microneedle cosmetic products and the integration of advanced materials, such as biodegradable polymers and bioactive compounds, into device design. The period from 2025 to 2030 is expected to witness a compound annual growth rate (CAGR) in the high single digits to low double digits, reflecting both rising consumer acceptance and ongoing technological innovation. Industry sources and company disclosures suggest that the global market size could surpass several billion USD by 2030, with the Asia-Pacific region projected to exhibit the fastest growth due to its large consumer base and rapid adoption of novel beauty technologies.
- North America and Europe: Continued leadership in regulatory approvals and premium product launches.
- Asia-Pacific: Fastest CAGR, driven by consumer trends and local manufacturing capabilities.
- Key drivers: Minimally invasive procedures, home-use devices, and integration of active cosmetic ingredients.
Overall, the outlook for microneedle cosmetic delivery systems from 2025 to 2030 is highly positive, with established and emerging companies alike investing in scalable manufacturing and global distribution to meet escalating demand.
Technological Innovations: Next-Gen Microneedle Designs and Materials
The landscape of microneedle cosmetic delivery systems is rapidly evolving, with 2025 poised to witness significant technological advancements in both design and materials. Microneedles—tiny, minimally invasive projections—are increasingly being engineered to enhance the transdermal delivery of active cosmetic ingredients, such as hyaluronic acid, peptides, and vitamins, directly into the skin’s deeper layers. This approach offers improved efficacy over traditional topical applications, driving strong interest from both established cosmetic giants and innovative startups.
A key trend in 2025 is the shift toward dissolvable and biodegradable microneedle arrays. These systems, often fabricated from biocompatible polymers or sugars, dissolve upon skin insertion, releasing their payload without leaving behind sharp waste. Companies like LG Household & Health Care have commercialized dissolving microneedle patches for anti-aging and skin-brightening, leveraging proprietary formulations to optimize ingredient stability and skin absorption. Similarly, Amorepacific Corporation continues to expand its microneedle product lines, focusing on precision delivery and user comfort.
Material innovation is another focal point. Researchers and manufacturers are exploring next-generation polymers, such as hyaluronic acid derivatives and cross-linked polysaccharides, to improve mechanical strength and dissolution rates. 3M, a global leader in transdermal technologies, is actively developing advanced microneedle platforms that integrate novel materials for enhanced performance and scalability. These innovations are expected to facilitate the delivery of larger molecules and more complex formulations, broadening the range of treatable cosmetic concerns.
Design improvements are also accelerating. The latest microneedle arrays feature optimized geometries—such as varying needle lengths, densities, and tip shapes—to maximize skin penetration while minimizing discomfort. Digital manufacturing techniques, including micro-molding and 3D printing, are enabling the production of highly customized patches tailored to individual skin types and treatment areas. Companies like LTS Lohmann Therapie-Systeme AG are at the forefront of scalable manufacturing solutions, supporting the transition from laboratory prototypes to mass-market products.
Looking ahead, the integration of smart technologies—such as sensors for real-time skin monitoring and controlled release mechanisms—is anticipated to further differentiate next-gen microneedle systems. As regulatory frameworks mature and consumer demand for non-invasive, effective cosmetic treatments grows, the sector is expected to see robust expansion and increased collaboration between material scientists, device engineers, and cosmetic brands through 2025 and beyond.
Competitive Landscape: Leading Companies and Strategic Partnerships
The competitive landscape for microneedle cosmetic delivery systems in 2025 is characterized by a dynamic mix of established pharmaceutical and cosmetic companies, innovative startups, and strategic collaborations aimed at accelerating product development and market penetration. The sector is witnessing increased investment and partnership activity as companies seek to leverage microneedle technology for enhanced transdermal delivery of active cosmetic ingredients, such as hyaluronic acid, peptides, and vitamins.
Among the leading players, LG Chem stands out with its robust R&D pipeline and commercialized microneedle patch products targeting anti-aging and skin-brightening applications. The company has invested heavily in proprietary microneedle fabrication techniques and has formed alliances with global cosmetic brands to expand its reach in Asia and beyond. Similarly, L'Oréal continues to advance its microneedle-based skincare solutions, leveraging its in-house dermatological research and open innovation initiatives. L’Oréal’s partnerships with biotech firms and academic institutions are expected to yield new product launches in the next few years, particularly in the premium skincare segment.
In the United States, 3M maintains a strong presence through its Drug Delivery Systems division, which supplies microneedle platforms for both pharmaceutical and cosmetic applications. 3M’s expertise in scalable manufacturing and regulatory compliance positions it as a preferred partner for cosmetic brands seeking to commercialize microneedle products globally. Meanwhile, AbbVie (through its Allergan Aesthetics division) is exploring microneedle technology for minimally invasive cosmetic treatments, with ongoing collaborations aimed at integrating microneedle patches into its portfolio of aesthetic solutions.
Asian companies are particularly active in this space. CosMED Pharmaceutical (Japan) has developed dissolving microneedle patches for cosmetic use, focusing on high-efficacy delivery of active ingredients. The company has entered into distribution agreements with major beauty retailers in Japan and South Korea, and is expanding into Southeast Asia. In South Korea, Rion is recognized for its innovative hydrogel-based microneedle patches, which have gained traction in both domestic and international markets.
Strategic partnerships are a defining feature of the current landscape. Companies are increasingly collaborating with material science firms, dermatology clinics, and contract manufacturers to accelerate product development and ensure regulatory compliance. The next few years are expected to see further consolidation, with multinational cosmetic brands acquiring or partnering with microneedle technology specialists to secure competitive advantages in the rapidly growing market for advanced cosmetic delivery systems.
Regulatory Environment: Global Standards and Compliance Challenges
The regulatory environment for microneedle cosmetic delivery systems is rapidly evolving as these technologies gain traction in the global beauty and personal care market. As of 2025, regulatory agencies are working to clarify standards and compliance requirements, given the unique nature of microneedle devices, which straddle the line between cosmetics and medical devices. This duality presents significant challenges for manufacturers and brands seeking to commercialize microneedle-based products worldwide.
In the United States, the U.S. Food and Drug Administration (FDA) distinguishes between cosmetic and medical microneedle products based on intended use and claims. Cosmetic microneedle patches that deliver non-drug ingredients (such as hyaluronic acid or peptides) are generally regulated as cosmetics, provided they do not penetrate beyond the stratum corneum or make therapeutic claims. However, if a product is intended to treat or prevent disease, or delivers active pharmaceutical ingredients, it is classified as a medical device or drug, triggering more stringent premarket requirements. The FDA has issued guidance on microneedle devices, but ongoing industry feedback suggests a need for clearer, more specific standards for cosmetic applications.
In the European Union, the European Medicines Agency (EMA) and national competent authorities oversee the regulation of microneedle products. The EU’s Medical Device Regulation (MDR) 2017/745, fully enforced since 2021, has increased scrutiny of borderline products. Cosmetic microneedle patches must not compromise skin integrity or deliver substances into the living layers of the skin to remain classified as cosmetics. The Cosmetics Europe industry association is actively engaging with regulators to develop harmonized guidelines, as the lack of uniform definitions and testing protocols creates compliance uncertainty for manufacturers.
In Asia, regulatory approaches vary. In South Korea, a global leader in microneedle cosmetic innovation, the Ministry of Food and Drug Safety (MFDS) has established specific guidelines for microneedle patches, requiring safety and efficacy data for both domestic and imported products. China’s National Medical Products Administration (NMPA) is also increasing oversight, with new requirements for product registration and post-market surveillance.
Looking ahead, the next few years are expected to bring greater regulatory harmonization, driven by industry collaboration and the need to facilitate international trade. Leading manufacturers such as LG Household & Health Care and Amorepacific are investing in compliance infrastructure and working with global regulators to shape future standards. However, challenges remain, particularly in defining the boundary between cosmetic and therapeutic use, and in establishing validated testing methods for microneedle safety and performance. As the market matures, regulatory clarity will be critical to ensuring consumer safety and supporting continued innovation in microneedle cosmetic delivery systems.
Consumer Adoption: Shifting Preferences and Demand Drivers
Consumer adoption of microneedle cosmetic delivery systems is accelerating in 2025, driven by a convergence of technological innovation, shifting beauty preferences, and heightened demand for minimally invasive skincare solutions. Microneedle patches and rollers, which deliver active ingredients such as hyaluronic acid, peptides, and vitamins directly into the skin, are increasingly viewed as effective alternatives to traditional topical creams and more invasive procedures.
A key driver of this trend is the growing consumer desire for at-home, professional-grade skincare. The COVID-19 pandemic catalyzed a shift toward self-administered beauty treatments, and this preference has persisted, with consumers seeking convenience, efficacy, and safety. Microneedle systems, which promise enhanced ingredient penetration and visible results with minimal discomfort, align well with these expectations. Companies such as L'Oréal have invested heavily in research and development of microneedle patches, with their SkinCeuticals and La Roche-Posay brands exploring targeted delivery for anti-aging and brightening applications.
Another significant factor is the increasing transparency and education around cosmetic science. Consumers are more informed about the limitations of conventional topical products and are seeking evidence-based solutions. The rise of social media and beauty influencers has amplified awareness of microneedle technology, with visible before-and-after results fueling demand. In response, established skincare brands and device manufacturers are expanding their portfolios. AbbVie, through its Allergan Aesthetics division, has signaled interest in integrating microneedle delivery into its aesthetic product lines, reflecting broader industry momentum.
The market is also witnessing the entry of specialized device manufacturers such as Renophase and Dermaroller GmbH, which offer both professional and consumer-grade microneedle systems. These companies emphasize product safety, sterility, and regulatory compliance, addressing consumer concerns about device quality and skin health. Additionally, partnerships between cosmetic brands and technology developers are expected to proliferate, further accelerating innovation and adoption.
Looking ahead, the outlook for microneedle cosmetic delivery systems remains robust. As regulatory frameworks evolve and clinical evidence supporting efficacy grows, consumer trust is likely to strengthen. The next few years are expected to see broader product availability, greater personalization (such as ingredient customization), and integration with digital skin analysis tools. This positions microneedle technology as a central pillar in the future of advanced, consumer-driven skincare.
Application Areas: Skincare, Anti-Aging, and Beyond
Microneedle cosmetic delivery systems are rapidly transforming the landscape of skincare, anti-aging, and adjacent beauty applications as of 2025. These minimally invasive devices, which create microchannels in the skin to enhance the penetration of active ingredients, are increasingly favored for their efficacy, safety, and consumer convenience. The technology is being adopted by both established cosmetic giants and innovative startups, with a focus on delivering peptides, hyaluronic acid, vitamins, and other bioactives directly into the dermis for improved results.
In the skincare sector, microneedle patches and rollers are now widely available for at-home use, targeting concerns such as fine lines, hyperpigmentation, and acne scars. Companies like L'Oréal have invested heavily in microneedle research, launching products that utilize dissolvable microneedles loaded with anti-aging compounds. L'Oréal’s partnership with biotech firms has accelerated the development of personalized microneedle patches, tailored to individual skin profiles, which are expected to reach broader markets in the next few years.
Another major player, Shiseido Company, Limited, has introduced microneedle patches in Asia and is expanding their reach globally. Their products focus on delivering hyaluronic acid and retinol, with clinical data supporting improvements in skin hydration and wrinkle reduction. Shiseido’s ongoing R&D aims to incorporate additional actives, such as growth factors and antioxidants, to address a wider range of skin concerns.
Beyond anti-aging, microneedle systems are being explored for applications such as hyperpigmentation, scar reduction, and even hair regrowth. AbbVie, through its Allergan Aesthetics division, is investigating microneedle-assisted delivery of botulinum toxin and other injectables, potentially offering less painful and more precise alternatives to traditional injections. This could significantly expand the use of microneedle technology in both clinical and consumer settings by 2026 and beyond.
The outlook for microneedle cosmetic delivery systems is robust, with ongoing advancements in materials science—such as biodegradable polymers and smart-release mechanisms—poised to further enhance efficacy and safety. Regulatory acceptance is also increasing, as demonstrated by the growing number of products receiving approvals in key markets. As consumer demand for non-invasive, effective skincare solutions continues to rise, microneedle technology is expected to play a central role in the evolution of cosmetic and dermatological treatments over the next several years.
Supply Chain and Manufacturing Advances
The supply chain and manufacturing landscape for microneedle cosmetic delivery systems is undergoing significant transformation as the sector matures and demand accelerates in 2025. Key drivers include the need for scalable, high-throughput production, stringent quality control, and the integration of advanced materials to meet both regulatory and consumer expectations.
Major industry players are investing in automated manufacturing lines to address the growing market for microneedle patches and devices. For example, LTS Lohmann Therapie-Systeme AG, a global leader in transdermal and microneedle technologies, has expanded its production capabilities to support both pharmaceutical and cosmetic applications. Their facilities are equipped with precision micro-molding and roll-to-roll processing, enabling the mass production of dissolvable and solid microneedle arrays with consistent quality.
Material innovation is another focal point. Companies such as 3M are leveraging their expertise in medical adhesives and polymers to develop microneedle platforms that are both biocompatible and suitable for cosmetic actives. 3M’s manufacturing infrastructure allows for rapid prototyping and scale-up, which is critical as cosmetic brands seek to launch new microneedle-based products with shorter development cycles.
In Asia, CosMED Pharmaceutical Co., Ltd. in Japan has become a prominent contract manufacturer for cosmetic microneedle patches, supplying both domestic and international brands. Their vertically integrated supply chain—from raw material sourcing to final packaging—enables tight control over product quality and traceability, which is increasingly demanded by global cosmetic companies.
Supply chain resilience is also being addressed through strategic partnerships and regional diversification. For instance, several European and North American brands are collaborating with specialized microneedle manufacturers to localize production and reduce lead times, mitigating risks associated with global logistics disruptions.
Looking ahead, the next few years are expected to see further automation, with the adoption of Industry 4.0 technologies such as real-time process monitoring and AI-driven quality assurance. This will not only improve yield and reduce costs but also support the customization of microneedle patches for specific cosmetic applications. As regulatory frameworks for cosmetic microneedle products become more defined, manufacturers are investing in compliance-ready facilities and documentation systems to ensure smooth market entry worldwide.
Overall, the supply chain and manufacturing advances in 2025 are setting the stage for broader adoption of microneedle cosmetic delivery systems, with scalability, quality, and innovation at the forefront.
Sustainability and Biocompatibility in Microneedle Systems
Sustainability and biocompatibility are rapidly becoming central considerations in the development and commercialization of microneedle cosmetic delivery systems as the sector matures into 2025 and beyond. The cosmetic industry’s increasing focus on eco-friendly materials and safe, skin-compatible formulations is driving innovation in both microneedle fabrication and the active ingredients they deliver.
A key trend is the shift from traditional silicon or metal microneedles to biodegradable polymers and naturally derived materials. Companies such as LG Chem and Amorepacific—both major players in the South Korean beauty and materials sectors—have been at the forefront of developing dissolvable microneedle patches using hyaluronic acid and other biocompatible polymers. These materials not only reduce the risk of skin irritation and allergic reactions but also eliminate the need for post-use disposal of sharp waste, addressing environmental and safety concerns.
In 2024 and into 2025, LG Chem has expanded its portfolio of microneedle patches for cosmetic applications, emphasizing the use of plant-based and biodegradable polymers. Their research and product launches highlight a commitment to minimizing environmental impact while maintaining efficacy in transdermal delivery of active ingredients such as peptides, vitamins, and hyaluronic acid. Similarly, Amorepacific has continued to invest in R&D for microneedle systems that dissolve completely after application, leaving no residue and reducing the environmental footprint of single-use cosmetic products.
Another notable development is the integration of green manufacturing processes. Companies are increasingly adopting solvent-free or water-based fabrication techniques to further reduce the environmental impact of microneedle production. This aligns with broader industry goals for sustainability and regulatory trends favoring eco-friendly cosmetic products.
Biocompatibility remains a top priority, with rigorous in vitro and in vivo testing protocols being implemented to ensure that microneedle materials and delivered actives are safe for repeated use on human skin. The use of naturally derived polymers, such as those based on hyaluronic acid, chitosan, and cellulose, is expected to grow, as these materials are not only biodegradable but also well-tolerated by the skin.
Looking ahead, the outlook for sustainable and biocompatible microneedle cosmetic delivery systems is strong. As consumer demand for green beauty products intensifies and regulatory scrutiny increases, manufacturers are likely to accelerate the adoption of renewable materials and closed-loop production systems. The next few years will likely see further collaboration between material science innovators and leading cosmetic brands, with companies like LG Chem and Amorepacific setting industry benchmarks for both sustainability and skin safety in microneedle technology.
Future Outlook: Opportunities, Challenges, and Strategic Recommendations
The future outlook for microneedle cosmetic delivery systems in 2025 and the coming years is marked by both significant opportunities and notable challenges. As the global demand for minimally invasive cosmetic procedures continues to rise, microneedle technologies are positioned to play a transformative role in the skincare and beauty industry. These systems offer enhanced transdermal delivery of active ingredients, improved patient compliance, and reduced downtime compared to traditional methods.
Key industry players are accelerating innovation and commercialization. LG Chem, a major South Korean chemical and biotechnology company, has expanded its microneedle patch portfolio, focusing on anti-aging and skin-brightening applications. Similarly, AbbVie (through its Allergan Aesthetics division) is exploring microneedle-based delivery for botulinum toxin and other cosmetic actives, aiming to offer less invasive alternatives to injectables. 3M, a global leader in medical adhesives and transdermal technologies, continues to invest in microneedle platforms, leveraging its expertise in skin-interfacing materials to develop next-generation cosmetic patches.
Opportunities in the sector are driven by consumer preferences for at-home treatments and personalized skincare. The rise of direct-to-consumer brands and e-commerce is enabling rapid adoption of microneedle patches for issues such as wrinkles, hyperpigmentation, and acne. Companies like International Research Laboratories (known for the RapidRenew Skin Perfecting Polish) and L'Oréal are actively developing and marketing microneedle-based products, with L’Oréal investing in research partnerships to optimize ingredient delivery and user experience.
However, challenges remain. Regulatory pathways for cosmetic microneedle devices are evolving, with agencies such as the U.S. FDA and the European Medicines Agency scrutinizing claims and safety data. Ensuring consistent manufacturing quality, sterility, and biocompatibility is critical, especially as products transition from clinical to consumer markets. Intellectual property disputes and the need for robust clinical evidence to support efficacy claims may also slow market entry for some innovations.
Strategic recommendations for stakeholders include investing in advanced manufacturing technologies to ensure scalability and quality, fostering collaborations with dermatologists and regulatory experts, and prioritizing consumer education to build trust in microneedle-based solutions. Companies that can demonstrate both safety and superior cosmetic outcomes are likely to capture significant market share as the segment matures. The next few years will be pivotal as the industry navigates regulatory landscapes and consumer expectations, with leading firms such as LG Chem, 3M, and L'Oréal setting the pace for innovation and commercialization.
Sources & References
- LTS Lohmann Therapie-Systeme AG
- NanoPass Technologies
- L'Oréal
- Shiseido Company, Limited
- LTS Lohmann Therapie-Systeme AG
- Nissha Co., Ltd.
- Amorepacific Corporation
- European Medicines Agency
- Cosmetics Europe
- Ministry of Food and Drug Safety
- Renophase
- Dermaroller GmbH